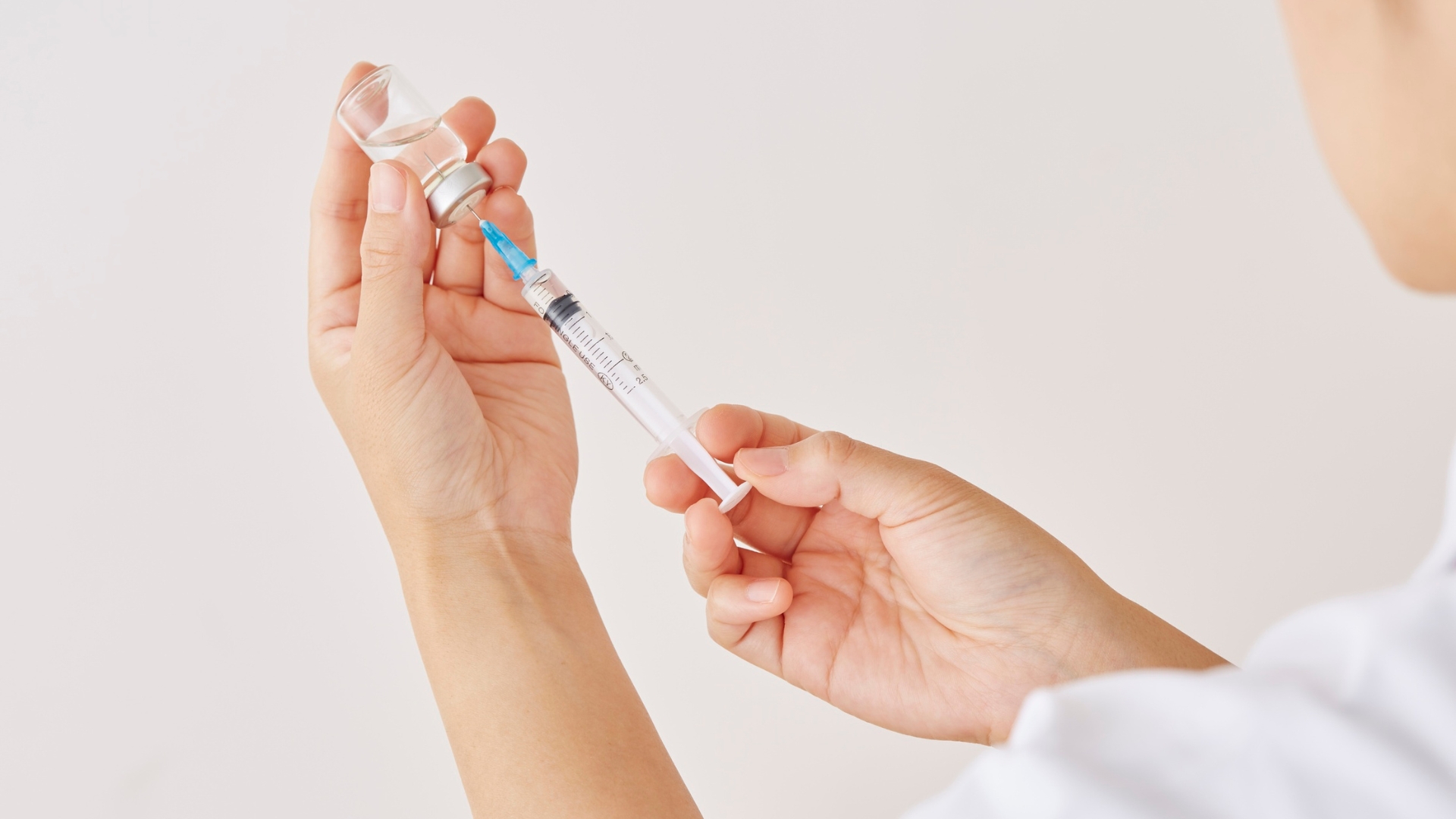
In response to the UK Health Security Agency’s (UKHSA) recent warning over a concerning cluster of botulism cases linked to counterfeit botulinum toxin, a comprehensive new safety guide – The Invisible Risk Behind Unregulated Botulinum Toxin (Botox Like) Treatments – has been launched to educate and protect patients considering aesthetic treatments.
Between 4 June and 14 July 2025, the UKHSA confirmed 38 cases of iatrogenic botulism in England, all traced to unlicensed or counterfeit botulinum toxin injections administered in non-clinical settings. The outbreak has been widely reported in the national media and has triggered industry-wide concern and renewed calls for tighter regulation and public awareness.
Botulism, a rare but life-threatening condition caused by Clostridium botulinum toxins, can lead to symptoms ranging from drooping eyelids and blurred vision to paralysis of the respiratory muscles. In this recent outbreak, six patients required critical care for respiratory complications – a stark reminder of the serious risks posed by improperly sourced or administered injectables.
Written by award-winning aesthetic nurse and educator Julie Scott, supported by the Joint Council for Cosmetic Practitioners (JCCP), the newly published guide outlines the steps patients must take to safeguard themselves when undergoing botulinum toxin treatments.
Scott emphasises that unregulated practitioners and counterfeit toxins bypass vital safety protocols, posing “a silent but significant threat to public health.” She adds:
“This guide empowers patients to make informed, confident decisions. It’s about shifting the narrative from aesthetic trends to safe, evidence-based practice.”
The guide’s release follows a joint statement from the JCCP, Save Face, BCAM and BAMAN urging consumers to only seek treatments from qualified medical professionals in clinical environments using MHRA-approved products.
With public health and safety in sharp focus, this guide acts as a critical tool to help people spot red flags, ask the right questions, and avoid potentially life-altering outcomes. It also supports the wider push for mandatory licensing of aesthetic practitioners and premises – a priority long championed by regulatory and professional bodies, as well as Hamilton Fraser.
To read and download the full guide, click here.
You can listen to our latest podcast on regulation with Andrew Rankin, here.